Safety and efficacy in a monocentric, open - label study. “The efficacy of polyvinylpyrrolidone-zinc gluconate and taurine gel (gelX®) in the prophylaxis and treatment of oral mucositis in children undergoing chemotherapy”.
Study involving 15 patients treated with gelX® Oral Spray. Poster presented at the EHA conference, June 11 - 14, 2015, Vienna
Summary: E1179
Typography: Presentation of an electronic poster
BACKGROUND
The combination of polyvinylpyrrolidone-zinc gluconate and taurine (gelX®) is an oral lubricating gel used in the treatment of oral mucositis (SB). Oral mucositis is a major problem for doctors and nurses as a secondary complication due to the administration of chemotherapy, which is accompanied by mouth pain and reduced oral intake, difficulty in digestion, especially in young children, with a significant impact on the quality of life and in the alimentary state. GelX® forms a protective barrier that adheres and overlaps the mucous membranes of the mouth, thereby protecting sensitive nerve endings and lubricating the oral tissue.
AIM OF THE STUDY
Evaluation of the effectiveness of gelX® for prophylaxis or treatment in children receiving chemotherapy for malignant haematological diseases.
METHODS
We conducted a prospective monocentric observational study in children with acute leukemia undergoing chemotherapy in accordance with the BFM protocols. Parents signed a consensual statement after being informed. The study did not receive sponsorship support. In the prophylactic use arm, nursing care of the oral cavity with gelX® started on the first day of chemotherapy and continued until the onset of the neutropenia stage. In the therapeutic use arm, oral cavity care started on the first day of chemotherapy with other oral products and continued with gelX® after the onset of oral mucositis. The use of the product continued until the oral mucositis regressed. The maximum recommended dose of oral products was three times a day, according to the recommendations of the product. Oral mucositis was controlled daily by doctors using the WHO 0-4 scale (0 = no, 1 = inflammation or erythema, 2 = erythema, ulcer, possibility of swallowing solid foods, 3 = ulcer with extensive erythema and inability to swallow solid foods , 4 = extensive mucositis in degree of inability to feed). We also gathered data on how patients rated pain (diffuse pain, mouth pain, neck pain) using the VAS 1-5 scale (1 = no pain, 2 = mild pain, 3 = moderate pain, 4 = severe pain, 5 = extremely high pain), and scale VAS to evaluate saliva production for a) swallowing (1 = normal, 2 = inability to swallow certain solid foods, 3 = inability to swallow soft foods, 4 = liquid ingestion ability only, 5 = inability to swallow), b) the degree of VAS to evaluate saliva production for a) (1 = normal sal- ivation, 2 = mild reduction of salivation, 3 = moderate reduction of salivation, 4 = significant reduction of salivation, 5 = absence of salivation) and c) , 3 = moderate viscosity increase, 4 = significant viscosity increase, 5 = drying saliva in mouth or on the lips). The tolerability of oral lubricants was evaluated on a daily basis by patients or parents using 1-5 on the VAS scale: 1 = tolerable without any problems, 2 = satisfactory, 3 = indifferent, 4 = unsatisfactory, 5 = unacceptable . The reduction in pain associated with oral mucositis and improved food intake were assessed by patients / parents using the 1-5 score on the VAS scale (1 = excellent, 2 = good, 3 = mild, 4 = almost no effect, 5 = no effect in general). Statistical univariate analysis was performed using a Fisher Precision Statistical Program statistic. Statistically significant differences were defined as p <0.05.
RESULTS
A total of 15 patients participated in the analysis, of which 8 were in the prophylaxis arm (group A) and 7 in the treatment arm (group B). The characteristics of the groups are presented in the table below. The highest degree of oral mucositis was most likely in group B versus group A, p = 0.018. Patient / parent scores for oral pain (p = 0.01) and saliva (p = 0.033) were significantly lower in the prophylaxis group. We also noticed a significant difference in the reduction of pain related to oral mucositis (p = 0.001), oral intake (p = 0.001) and the use of analgesics (p = 0.005). No differences in mean tolerance for oral lubricants versus gelX® in groups A and B were observed.
COMPOSITION
There is limited data on the use of gelX® as a prophylactic or therapeutic agent in children. In our study, oral spray was given for both prophylactic and therapeutic purposes. Data gathered show improved results in the development of oral mucositis and the quality of life in the prophylactic arm. Key words: chemotherapy toxicity, children, mucositis.
| Precautionary arm (group A) | Precautionary arm (group B) | P= | |
| Number of patients | 8 | 7 | - |
| Average age in years (range) | 5,5 (2 - 10) | 4, (0,75 - 5) | - |
| Sex: I/O | 3/5 | 1/6 | 0,287 |
| Diagnosis: Acute lymphoblastic leukemia / Acute myelogenous leukemia | 5/3 | 6/1 | 0,287 |
| Maximum degree of SB (range) | 1, (1 - 3) | 4, (3 - 5) | 0,018 |
| - Diffuse pain | 1, (1 - 2) | 2, (2 - 3) | 0,123 |
| - Pain in the mouth | 1, (1 - 3) | 4, (3 - 5) | 0,001 |
| - Sore throat | 1, (1 - 2) | 3, (1 - 4) | 0,369 |
| - Ingestion | 1, (1 - 4) | 3, (1 - 4) | 0,304 |
| - Quantity of saliva | 1, (1 - 2) | 2, (1 - 5) | 0,091 |
| - Recommendation | 1, (1 - 4) | 2, (1 - 5) | 0,033 |
| Average tolerance of oral lubricants (range) | 1, (1 - 2) | 2, (4 - 8) | 0,369 |
| Average daily reduction in AB pain | 2, (0 - 4) | 7, (4 - 8) | 0,001 |
| An average daily improvement in oral intake | 2, (0 - 3) | 8, (5 - 10) | 0,001 |
| Systemic analgesic therapy with painkillers | 2/8 | 7/7 | 0,005 |
A monocentric, open-label study of safety and efficacy in 40 patients treated with GelX Oral Gel and gelX® Oral Spray. Presented at the AEMM confernce, 23 - 25 October 2015, Rome
Relief from the pain of oral mucositis with GelX Oral Gel and gelX® Oral Spray (GOG & GOS) Zannier F, Belloni P, Toniolo D, Cozzi C, A.O.G. Salvini Rho (MI).
MATERIALS AND METHODS
We evaluated 40 patients with solid tumors who had to undergo chemotherapy containing cisplatin / 5FU, chemo-radiotherapy and radiotherapy. Each patient used: GOG for prophylaxis, twice daily, prior to anticancer therapy and GOS as a treatment, three times a day during anti-cancer therapy. SB was assessed by the physician and the patient using the RTOG scale at the beginning of the prophylactic treatment, after one month of treatment, at the end of the treatment and one month after the end of the treatment.
RESULTS
One month after the end of treatment, 30 patients experienced complete regression of the pain and swelling, 8 patients had no pain but swelling, 2 patients had mild pain and no swelling.
CONCLUSIONS
GOG & GOS is safe and effective and could be a new approach to managing and preventing CB. Phase III of the study with regard to treatment preferred by doctors is underway.
The protective action of gingival mucositis: pilot, double-blind study with GelX Oral Gel (BMG0901) and placebo (Catania University, Italy) Doctor of Orthodontic Research, coordinator Prof. Caltabiano M., University of Catania, Italy.
MATERIALS AND METHODS
In dental surgery, for a period of eleven months, a total of 60 patients aged between 18 and 71 had stomatitis. Following information and subsequent consensus, these patients were randomized into two groups of 30 subjects each:
- Group A: patients who did not receive any treatment as a control group
- Group B: patients treated with BMG0901
For the patients treated, the recommended dose of the study product was approximately 2 - 3 times a day (after main meals and in the evening before bedtime). Patients were instructed not to eat or drink for at least one hour after application. They were also asked to cover the entire inflamed surface with the gel.
Improvement of clinical signs was evaluated on the basis of the following criteria:
- The presence of oral ulcers
- Number and distribution of inflamed surfaces
The following conditions were defined as healing:
- No symptoms of pain or burning
- No ulceration
To evaluate the efficacy of the products, the following scale was used one month after treatment:
- Complete healing (+++)
- Presence of lesions but asymptomatic (++)
- Reduce symptoms by half (+)
- Continuation of symptoms and indications (-)
- Undesirable effects (^)
RESULTS AND DISCUSSION
The results recorded in the individual groups are presented in the table and graphics below. No adverse events were observed in the treated patients and no local lesions were found in the mucosal treated locally.
| Group Α | Group Β | |
| Complete healing (+++) | 40% | 90% |
| Presence of lesions but asymptomatic (++) | 20% | 5% |
| Reduce symptoms by half (+) | 10% | 5% |
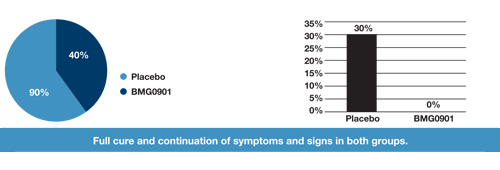
| Continuation of symptoms and indications (-) | 30% | 0 |
| Undesirable effects (^) | 0 | 0 |
The polyvinylpyrrolidone (PVP) contained as the main ingredient in BMG0901 forms a high molecular weight protective barrier contained in its composition and forms a distinct coating for the natural protection of the stomatitis. In addition, its combination of zinc and taurine guarantees the essential soothing effect of the composition, reducing the sense of focused irritation caused by irritation of the oral cavity. It is noted that PVP is a substance widely recognized for its ability to form a protective film and is inactive in the physiology of the human body. It exhibits a high level of compatibility with the majority of inorganic salts and natural and synthetic resins contained in either solutions or membranes as well as other chemical compounds.

